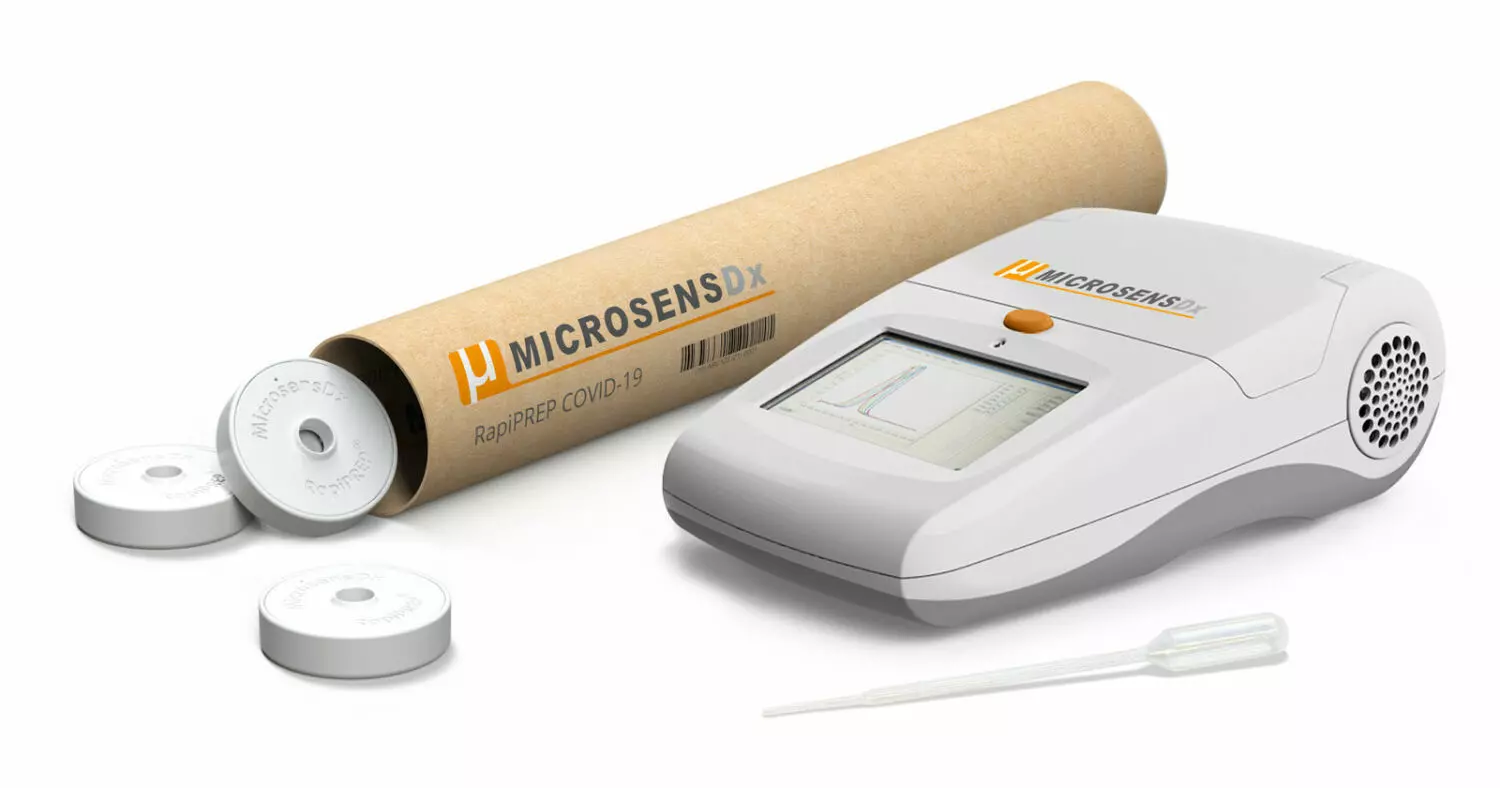
Renfrew Group assist MicrosensDx with the design and development of their Coronavirus COVID-19 RapiPrep® test cartridge.
Coronavirus testing kit
MicrosensDx announces a portable coronavirus testing kit for research use that takes less than 25 minutes from taking a sample to test result.
With the imminent threat of the coronavirus (SARS-CoV-2) forcing countries worldwide into lockdown and putting a severe strain on healthcare services, MicrosensDx has responded by adapting its current range of infectious disease rapid detection kits to create a coronavirus detection product, suitable initially for research use.
The MicrosensDx RapiPrep®
The MicrosensDx RapiPrep® system enables highly sensitive SARS-CoV-2 detection in less than 25 minutes from taking a sample to test result. The portable, desktop kit will be used initially in scientific laboratories involved in vaccine and therapeutic research and development.
MicrosensDx is now engaged in a programme to gain CE accreditation for the SARS-CoV-2 test which will permit its sale and use in the diagnosis of the COVID-19 disease. The CE-marked kits will provide rapid ‘on demand’ testing support alongside the large-scale hospital laboratory testing instruments and will also allow disease testing to take place in facilities such as clinics, doctor’s offices and in the workplace.