The ‘Magic’ (MAGnetic resonance Imaging in paediatric Constipation) programme has been designed by experts at the birthplace of MRI – the University of Nottingham. The study involves gastroenterology experts from Nottingham University Hospitals NHS Trust and the NIHR Nottingham Biomedical Research Centre and is funded by the NIHR.
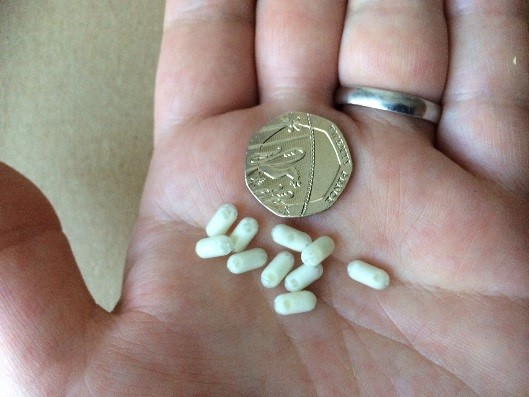 MRI-friendly ‘Magic Beans’
MRI-friendly ‘Magic Beans’
The ‘Magic Bean’ mini-capsules are made of a medical-grade plastic shell and are smaller than Tic Tacs to make them easy for children and young people to swallow.
They do not dissolve in the gut, and are filled with a MRI-visible liquid that stands out clearly in the scan pictures of the gut.
To test the imaging of these ‘magic beans’ the researchers initially used a crude model consisting of a string of sausages containing the capsules stuffed into a large supermarket chicken.
Leading the team is Associate Professor in Gastrointestinal MRI, Dr Luca Marciani, supported by lead clinician paediatric gastroenterologist David Devadason from the Nottingham Children’s Hospital.

Chronic constipation can seriously affect the health and quality of life of some children and young people and management of the problem is historically difficult as it is mostly based on symptom reports. Knowing how long it takes for food to travel through the gut could help clinicians direct treatment.
“This however is not very often tested in these patients since current X-ray methods do not show a clear picture of the colon anatomy due to overlapping colon loops. X-rays also deliver a radiation dose, which is not ideal for young patients.”
Dr Marciani added: “We wanted to design an accurate and objective way of measuring gut transit time to help doctors make a faster and more informed choice of treatment, for example whether to prescribe medicine that stimulates gut muscles or psychological therapy.
This could speed up treatment and improve outcomes for children and their families as well as saving money in the NHS. We are delighted that our Young Persons Advisory Group (YPAG) and a parent have been involved from the beginning and played an active part in the design of the capsules and the trial itself. They have also been invaluable in helping us create patient information sheets and even animated videos for YouTube.

“Constipation can be really painful. Sometimes it affects my normal activities and school. Taking part in this research is a really good activity to do because you know that you help a lot of people. I was happy to help.
Going in the MRI scanner was scary at the beginning but then it was fine. We did see some mini-capsules in my body, it was fun.
I would encourage other people to get involved in research, it is scary at the beginning but it’s a good cause and it helps a lot of people.”
Successful pilot study
After a pilot study in healthy adults, a group of 25 children and young people with constipation and a further 25 without constipation were recruited from the East Midlands to take part in the trial.
Three sets of 24 mini-capsules were given to each participant, to be swallowed with water or yoghurt, under supervision by a parent or guardian – one set every day for three consecutive days. On the fourth day they returned to the MRI department for a scan, which takes about 15 minutes.
The scans were carried out at the world famous Sir Peter Mansfield Imaging Centre on University Park in Nottingham. Further MRI scans were carried out on day 7 if any mini-capsules could be seen on the first set at day 4.
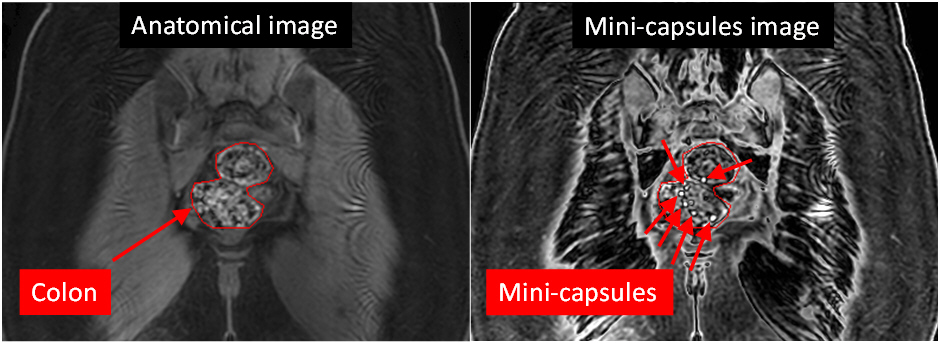
The results of the study so far are very positive, the mini-capsules were imaged successfully in the body, the study procedures were accepted well by the participants and the safety record was excellent.
New funding to expand the research
Now the funding has been extended with the award of a second NIHR i4i grant of £1.2 million to fund the second phase of the clinical trial MAGIC2. This will be a larger study in 436 young patients with constipation to show that using mini-capsules in standard clinical practice can improve the success of available treatments.
It will also develop new and more cost-effective ways to mass-produce the mini-capsules and create software capable to that can detect the mini-capsules automatically.

Help from industry partners
The researchers made the initial design of the mini-capsules with the help of the medical device design and technology innovation company Renfrew Group International in Leicester.
JEB Technologies, will now progress the MAGIC mini-capsules on to CE marking, manufacturing and commercialisation.
Motilent, a company specialising in the development of medical software for digestive disease, designed an initial software module for analysis of the mini-capsules’ images and will continue this work to ensure mini-capsule assessment is rapid and objective in clinical practice.
Medical supplies distributor Pentland Medical have advised the team on the potential market for the new mini-capsules and are partners in the new project which aims to end with commercialisation of the new mini-capsules medical device.
Wider collaboration
The multi-centre MAGIC2 clinical trial will be managed Derby Clinical Trials Support Unit (Derby CTSU), who are provisionally registered with the UKClinical Research Collaboration UKCRC), hosted by the University Hospitals of Derby & Burton NHS Foundation Trust.
The MAGIC2 team includes also specialsts from University College London, the University of Lincoln, the NIHR Children and Young People MedTech Co-operative in Sheffield, the East Midlands Academic Health Science Network (EMASHN), the Research Design Service (RDS) East Midlands (EM) and TRUSTECH in Manchester, the NIHR Clinical Research Network East Midlands and the NIHR Clinical Research Facility.
Story credits
More information and booking is available from Dr Luca Marciani via email luca.marciani@nottingham.ac.uk